Ewing sarcoma (brief information)
A Ewing sarcoma is a rare malignant tumour in childhood and adolescence that originates mostly in the bones. This text provides information about the characteristics of this disease, its frequencies, causes, symptoms, diagnosis, treatment, and prognosis.
Author: Maria Yiallouros, Editor: Maria Yiallouros, Reviewer: Prof. Dr. med. Uta Dirksen, English Translation: Dr. med. Gesche Riabowol (nee Tallen), Last modification: 2024/03/19 https://dx.doi.org/10.1591/poh.patinfo.ewing.kurz.20101215
Table of contents
General information on the disease
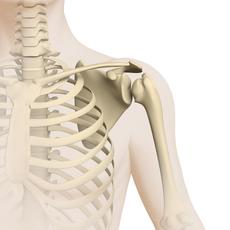
Ewing sarcomas are malignant, solid tumours that mostly arise within the bone tissue. The disease is named after the American cancer researcher Dr. James Ewing (1866-1943), who first described this tumour in 1921. Most Ewing’s sarcomas grow and spread very quickly. Without an appropriate treatment, the disease is always fatal.
In general, Ewing sarcoma can develop in any bone. However, pelvic bones, followed by the long bones of the upper or lower leg, the thoracic skeleton, the plate bones, and the spine are those most frequently affected.
The tumour may spread (metastasize) both within the bone and into the adjacent soft tissue. Rarely (about 15 %), Ewing sarcoma arises directly within soft tissue, that is to say, outside the bone and without the bone being involved. Such tumours are called extrasceletal or extraosseal Ewing sarcomas. Ewing sarcomas of pure soft tissue origin may be located in the kidneys, adrenal glands, lungs, or in the gastrointestinal tract.
Due to the rapid growth and spread of Ewing sarcomas, about 25 % of the children and adolescents with this disease already present with detectable daughter tumours (metastases) at the time of diagnosis, primarily in the lungs, but also in the bones and, less often, in the bone marrow. It has to be assumed for all other patients, too, that tumour cells have already spread elsewhere via the blood or lymphatic stream and, thus, have formed tiny metastases in other organs. These so-called micrometastases may not be detected at diagnosis due to their small size. Since Ewing sarcoma is thus considered a disease that may affect the whole body, it is also called a systemic disease.
Incidence
Following osteosarcomas, Ewing sarcomas are the second most common primary bone tumours in childhood and adolescence; they account for about 2 % of all pediatric malignancies. According to the German Childhood Cancer Registry (Mainz, Germany), approximately 50 children and adolescents under the age of 18 (about 3 of 1,000,000) are diagnosed with Ewing sarcoma in Germany per year.
Ewing sarcoma may develop at any age; however, the frequency of this type of cancer peaks in the second decade of life. In childhood and adolescence (age group 0-17 years), the median age at diagnosis is 13 years, with adolescents between 12 and 17 years of age being the most frequently affected. Nevertheless, Ewing sarcomas are also observed in babies, infants, and school children. Overall, boys and male adolescents are more frequently affected by the disease than girls (gender ratio: 1.3:1).
Histological characteristics and tumour types
Ewing sarcomas are primitive malignant tumours. It is still unknown as of today, from which precursor cell they arise. According to the most recent research, they develop from immature (undifferentiated) tissue cells, so-called mesenchymal stem cells, or from primitive neuroectodermal stem cells.
Ewing sarcoma cells are histologically assigned to the mesenchymal small-, blue- and round cell tumour types; they can only be differentiated from similar looking, undifferentiated tumour cells of other malignancies (such as neuroblastoma, medulloblastoma, Non-Hodgkin lymphoma, soft tissue sarcoma, small cell osteosarcoma and retinoblastoma) by immunohistochemical and molecular genetic analyses. Since these tumours are rare, the associated analyses are carried out in specialised laboratories (see chapter “Diagnosis”).
Until recently, different subtypes have been differentiated in the group of Ewing sarcomas, based on their histological features and the site of the primary tumour. These included the classic Ewing sarcoma (EWS), the peripheral malignant primitive neuroectodermal tumour (PPNET or pPNET), the Askin tumour of the chest wall and Ewing tumours of soft tissues. As per classification of the World Health Organization (WHO classification), all these tumours have been summarized to one entity, meaning that in general only the term “Ewing sarcoma” is used. All Ewing sarcomas are highly malignant.
Causes
The underlying causes for the development of a Ewing sarcoma are still unknown. Neither external factors, such as a previous radiation therapy, nor inherited genetic factors (genetic predisposition) seem to play a relevant role. However, the disease shows ethnical preference by developing more frequently in white-skinned people (Caucasians) than in people of colour (Asians or Africans).
It is also known that tumour cells of Ewing sarcomas present with certain chromosomal aberrations, which all include a certain gene on chromosome 22 – the so-called Ewing sarcoma gene (EWS gene). These aberrations develop due to an exchange of chromosomal parts (translocation), mostly between the EWS gene on chromosome 22 and a gene on chromosome 11. Accounting for 85 %, the most frequent translocation (so-called [t(11;22) (q24;q12) translocation]) is so specific for Ewing sarcoma that it allows confirmation of the diagnosis. For many Ewing sarcomas, additional genetic alterations have been identified (for example increased numbers of chromosomes or loss or gain of genetic material, respectively). The genetic defects resulting from those aberrations promote that a healthy cell transforms into a tumour cell. In general, those genetic defects, which are found in tumour tissue only, are not inherited.
Symptoms
The most frequent symptoms of Ewing sarcoma are pain and a swelling in the tumour region.
Pain may be intermittant and is usually activity-dependent, but frequently also occurs at rest, for example during night time. With increasing tumour growth, the pain may be accompanied by a visible and/or palpable lump at the tumour site. The swelling may be reddened and may sometimes lead to an impaired mobility, which initially may be mistaken for growing pains or a sports injury or some bone inflammation.
Since Ewing sarcomas may develop in any bone as well as in soft tissue, further symptoms are quite diverse, depending on the site and extent of the tumour. In case the spinal column and/or peripheral nerves (peripheral nervous system) are affected, deficits such as paralysis may be the major symptom. Tumours in the pelvic or chest region or in the thigh may remain unidentified for quite a while. About one third of patients complain of general symptoms such as fever, feeling of illness (malaise), weight loss and/or fatigue, which may be indicative of an advanced disease. For some patients, only a few weeks, for others up to several months may pass between first symptoms and the diagnosis of osteosarcoma.
Good to know: Not all children and adolescents presenting with the complaints described above suffer from Ewing sarcoma or any other malignant bone tumour. However, every type of musculoskeletal pain in a child or a teenager should be taken seriously and be dealt with by an experienced paediatrician in order to appropriately rule out an underlying cancer.
Diagnosis
If the paediatrician thinks that the young patient’s history (anamnesis) and physical examination are suspicious of a malignant bone tumour, the child should immediately be referred to a hospital with a childhood cancer program (paediatric oncology unit), where further diagnostics can be initiated and performed by childhood cancer professionals. Very close collaboration between various specialists (such as paediatric oncologists, paediatric surgeons, paediatric radiologists, to name a few) is required both to find out whether the patient really suffers from a malignant bone tumour and, if so, to determine the tumour type and the extension of the disease. Knowing these details is crucial for optimal treatment planning and prognosis.
Laboratory tests
The diagnosis of Ewing sarcoma requires, aside from a comprehensively taken history and physical exam, blood and urine tests. Although tumour markers that are specific for Ewing sarcoma have not been identified yet, certain peculiarities indicative of the type of the disease and/or differential diagnoses do exist, and can be discovered via laboratory testing.
Imaging tests to confirm tumour existence
Based on their typical radiological features, most malignant bone tumours can already be diagnosed by X-ray examination. Additional imaging procedures such as magnetic resonance imaging (MRI) and/or computed tomography (CT) with a contrast agent subsequently help to define the exact tumour site and size as well as its demarcation with regard to adjacent tissue (such as blood vessels, muscles, tendons or joints). Nearby metastases (called skip metastases) are easily detectable by these methods as well.
For imaging of affected soft tissue and bone marrow, MRI is superior to CT. Apart from plain x-rays, it is, therefore, considered as the gold standard in radiological diagnosis of a Ewing sarcoma. It also serves as a basis for planning subsequent surgery and for monitoring the course of the disease during chemotherapy. However, a CT may be additionally necessary in order to examine bone changes more closely.
Obtaining a tumour sample (biopsy)
For final confirmation of the diagnosis, tumour tissue is required to be removed (biopsy). The biopsy should only be performed by doctors who are specialized in surgery of sarcomas. This ensures that the access chosen for biopsy does not cause problems for subsequent treatment. An unfavourably planned biopsy may result in the requirement of a far more extensive subsequent surgery than initially necessary or, worst case scenario, in the inoperability of a tumour that would have been initially removable.
The required tissue samples are either gained during the surgical procedure for tumour resection (open surgery) or by needle biopsy. The latter uses special needles to punch multiple samples of tumour tissue. The obtained samples are subsequently analysed histologically, immunohistochemically and molecular genetically by specialists. Molecular genetic analysis has special relevance, since identification of genetic alterations that are typical for Ewing sarcomas confirm the diagnosis and allow ruling out other, similar tumour types (see chapter „Causes“).
Tests to assess tumour spread (staging)
Once the diagnosis of a Ewing sarcoma has been confirmed, further tests are performed to assess potential tumour spread into other organs (metastasis). In this context, diagnostic imaging plays a major role. At first, accurate measuring of the primary tumour is required (so-called volumetry), since its volume (and shrinking during treatment) has a major impact on the patient’s probability of survival.
In order to assess or, respectively, rule out lung metastases, a computed tomography of the chest (chest-CT) is performed. A skeletal scintigraphy (bone scan) using radioactively labelled technetium (99mTc) serves to detect or rule out bone metastases. Increasingly, positron emission tomography (PET) with radioactively labelled glucose (18F-fluoride-deoxyglucose, short FDG) is performed instead of a bone scan. This very sensitive nuclear medical imaging procedure is either combined with magnetic resonance imaging or computed tomography. Regardless of whether bone scan or PET are chosen, an MRI is also done for all clinically and radiologically suspicious regions. Some patients benefit from a whole-body MRI.
To find out whether the bone marrow is affected, tissue samples are obtained by bone marrow puncture and bone marrow punch biopsy and, subsequently, analysed histologically and molecular genetically. If the central nervous system (brain and spinal cord) is suspected to be affected as well, it may even be necessary to take a sample of cerebrospinal fluid (lumbar puncture).
Tests before treatment begins
Before treatment begins, further tests are needed in order to assess the condition of different organs. Therefore, the doctors will recommend an electrocardiography (ECG) as well as an ultrasound of the heart (echocardiography), a hearing test (audiometry), special diagnostics for determining kidney and lung functions as well as various blood tests. Any changes occurring during the course of treatment can be assessed and managed better based on the results of those initial tests, which thus help to keep the risk of certain treatment-related side effects as low as possible.
Good to know: Not all of the above-mentioned tests will be done for every single patient. On the other hand, additional tests not mentioned here may be required individually. Ask the doctor which diagnostics are necessary and why.
Treatment planning
After the diagnosis has been confirmed, therapy is planned. In order to design a highly individual, risk-adapted treatment regimen for the patient, certain individual factors influencing the patient’s prognosis (called risk factors or prognostic factors) are being considered before and during treatment (risk-adapted treatment strategy).
Important prognostic factors are the type, localisation, size and spread of the tumour (local or metastasised), which are assessed before treatment begins. In addition, the extent of surgical tumour/metastasis removal (imcomplete versus complete) as well as the response of the disease to chemotherapy have major impact on treatment planning. All these factors are included in treatment planning in order to achieve the best outcome possible for each patient.
Treatment
Treatment of children and adolescents with Ewing sarcoma should take place in a children's hospital with a paediatric oncology program. Only in such a childhood cancer centre, highly experienced and qualified staff (doctors, nurses and many more) is guaranteed, since they are specialized and focus on the diagnostics and treatment of children and teenagers with cancer according to the most advanced treatment concepts. The doctors in these centres collaborate closely with each other. Together, they treat their patients according to treatment plans (protocols) that are continuously optimised. Treatment in a centre with assigned sarcoma focus is considered optimal. Alternatively, the paediatric oncology program should collaborate with such a centre, in particular regarding surgery and radiotherapy.
The goal of the treatment is to achieve higher cure rates while avoiding side effects as much as possible.
Treatment methods
Treatment of children and adolescents with Ewing sarcoma consists of surgery and/or radiotherapy (local therapy) as well as chemotherapy. For some patients, a high-dose chemotherapy followed by autologous stem cell transplantation may be an option, too.
Radiotherapy (radiation therapy) is done using energy-rich, electromagnetic radiation, given through the skin to the tumour region. Radiation causes DNA damage in tumour cells, thereby leading to cell death. Chemotherapy uses drugs (so-called cytostatic agents or cytostatics) that can kill fast-dividing cells, such as cancer cells, or inhibit their growth, respectively.
Surgery and radiotherapy aim at maximally possible local disease control. Additional chemotherapy is necessary, because it appeared that though surgery and radiotherapy alone are capable of eliminating the tumour, metastases will almost always develop later on. Therefore a treatment is required – such as chemotherapy – that affects the whole body (so-called systemic treatment). However, chemotherapy alone cannot replace local therapy. Some patients with a high risk of recurrent disease may benefit from high-dose chemotherapy and subsequent autologous stem cell transplantation.
In order to prevent or adequately manage the side effects of the intensive therapy, specific supportive care (supportive therapy) regimens have been established. Here you will find information on supportive care as well as recommendations for home, the latter of which may be helpful during or after chemo- and radiotherapy.
Course of treatment
Usually, local therapy is provided between two cycles of chemotherapy. Overall, treatment takes about ten months, but – depending on many different factors – may also require more time. The different treatment phases will be outlined in the next paragraphs.
Chemotherapy prior to local therapy
Therapy is generally initiated by an intensive chemotherapy of several weeks (called induction chemotherapy or induction therapy). The goal of this chemotherapy is to shrink the tumour and potential spread (metastases) in order to optimise the conditions for subsequent surgical tumour removal, thereby contributing to safety and efficacy of surgery. Also, chemotherapy helps eliminate micrometastases (the tiny metastases that cannot be detected by imaging diagnostics), thus preventing further tumour spread.
In order to eliminate as many tumour cells as possible, patients receive a combination of different chemotherapeutic agents (cytostatics) that have proven to be effective in the treatment of Ewing sarcoma. These agents, administered by infusion, include vincristine, doxorubicin (= adriamycin), cyclophosphamide, ifosfamide, and etoposide (abbreviated “VDC/IE”). Chemotherapy is given as several courses over several (currently nine) days each, during which the patient needs to be inpatient. Between courses, patients can go home as outpatients. Readmission is only required in case of severe side-effects.
Local therapy – surgery and radiotherapy
Still during or latest after cessation of chemotherapy, local therapy is scheduled. The preferred treatment is surgery with the aim of gross total tumour removal. (This surgical procedure should definitely be performed in a specialised sarcoma centre.) A complete tumour removal, however, may not always be an option due to tumour location within functionally relevant body regions and, hence, radiation therapy will be considered instead or in addition. Some patients require a combination of surgery and radiotherapy, since the combined treatment lowers the risk of recurrent disease.
Which one of the two treatment methods is indicated or whether they will be combined depends on the patient and his / her disease condition and, thus, needs to be decided individually. Your caregiver team will inform you more in detail on the type and course of surgery or radiotherapy, respectively. Thanks to huge progress made for limb-sparing surgical techniques, it is fre-quently possible as of today to waive amputation for patients with large tu-mours of the arms or legs.
Following surgery, a pathologist examines the tumour tissue under the microscope to find out how the disease has responded to the preceding chemotherapy. This so-called histological response is assessed by measuring the amount of the tumour cells that are still alive. An amount of less than 10 % vital tumour cells is defined as good response, an amount of 10 % or more vital tumour cells as inadequate response. The grade of response (defined as grades 1-6) is considered when deciding on further treatment steps to be taken.
Good to know: as far as possible, metastases identified at the time of diagnosis are treated locally like the primary tumour, thus being surgically removed and/or irradiated.
Chemotherapy after local therapy
After local therapy,chemotherapy is continued (then called consolidation chemotherapy or consolidation therapy). Treatment intensity depends on tumour size and extent at diagnosis as well as on the tumour’s response to the chemotherapy preceding local therapy (induction chemotherapy).
Patients with local disease and poor response to the induction chemotherapy (more than 10 % living tumour cells) or with a large tumour (more than 200 ml of volume) will receive, after consolidation chemotherapy, if possible, a high-dose chemotherapy (with busulfan and melphalan) followed by an autologous stem cell transplantation. It has been shown that this treatment results in more favourable outcomes, but requires complete regression of the tumour (remission) after consolidation therapy.
If lung metastases exist already at the time of diagnosis and if these regress completely during consolidation (complete remission), additional radiation of both lungs is given. High-dose chemotherapy followed by autologous stem cell transplantation did not reveal any benefit compared to standard treatment in the context of studies. This also applies for patients with metastases other than lung metastases at diagnosis. They will therefore receive conventional chemotherapy; just for a subgroup of children under 14 years of age, high-dose chemotherapy with autologous stem cell transplantation may be of benefit.
Good to know: in some cases, additional radiation may be given after chemotherapy or high-dose chemotherapy.
Treatment in case of progressive or recurrent disease
Despite of optimised treatment methods, 30 to 40 % of patients with Ewing sarcoma will experience recurrent disease (relapse). There is no standardised treatment recommendation for these patients. Depending on the disease situation, multi-drug chemotherapy (for example with topoisomerase inhibitors such as etoposide, irinotecan or topotecan or alcylating agents (alkylants) like ifosfamide, cyclophosphamide and temozolomide), radiation therapy, surgical measures or a combination of these may be considered. Also, a high-dose chemotherapy may be an option after achievement of complete remission after relapse treatment.
When therapy with the goal of a cure is not an option any longer, maintenance of the patient’s life quality is of major importance, for example by providing pain control and maintaining functions (palliative care). In the context of phase-I/II studies it is attempted to improve the probabilities of survival for these patients, for example by introducing and testing new agents.
Therapy optimising trials and registries
In the large paediatriac treatment centres, children and adolescents with Ewing sarcoma receive therapy according to standardised treatment plans (protocols). These protocols are designed by experts and aim at steadily improving the patients’ survival rates while also reducing the risk of therapy-related late effects. Therapy according to such treatment protocols is usually carried out within therapy optimising trials or registries.
Therapy optimising trials are standardised and controlled clinical trials that aim at steadily developing and improving treatment concepts for sick patients based on the current scientific knowledge. Patients who cannot participate in any study, for example because none is available or open for them at that time or since they do not meet the required inclusion criteria, respectively, are often included in a so-called registry. One goal of a registry is to collect treatment data for research questions. Furthermore, the registry centre supports the doctors at site with (non-commital) treatment recommendations based on the most recent data on best treatment options, in order to provide the patient with optimal therapy even without the framework of a clinical study.
Since 2020, children and adolescents (as well as adults) with first diagnosis of Ewing sarcoma in Germany, who may not be treated in the context of any trial, can be registered with the International Euro Ewing Registry. The registry represents a continuation of the EWING 2008 registry, which was closed mid 2019. The study coordination centre for Germany is located at the Children’s Hospital of the University of Essen (principal investigator is Prof. Uta Dirksen, MD).
Since the end of 2018, patients with relapsed Ewing sarcoma are given the opportunity to participate in the international phase-II study rEECur. The rationale of this randomised controlled study is to compare four different chemotherapy regimens (topotecan and cyclophosphamide, irinotecan and temozolomide, gemcitabine and docetaxel; high-dose ifosfamide) in order to identify the most efficient one. Principal investigator of this trial is Dr. Martin McCabe (Birmingham). Study coordination centre for Germany is located at the Children’s Hospital of the University of Essen (principal investigator: Prof. Uta Dirksen, MD).
Aside from participating in this study, patients with recurrent disease can also register with the INFORM-Registry. The registry systematically records genetic changes in tumours with the goal to be able to offer individually tailored therapies, thereby improving outcomes for relapse patients. The abbreviation “INFORM” stands for INdividualised Therapy FOr Relapsed Malignancies in Childhood. The study coordination centre is located at the Hopp-Childhood tumour centre in Heidelberg (KiTZ) under supervision of Prof. Olaf Witt, MD, PhD.
Prognosis
The chances of survival (prognosis) for children and teenagers with Ewing sarcoma depend on various factors. In particular, the tumour’s response to preoperative chemotherapy as well as its site, size and extent at diagnosis are of major prognostic importance.
During the last decades, prognosis of children and teenagers with Ewing sarcoma has significantly been increased, thanks to modern diagnostic procedures and standardised combination therapies in the framework of therapy optimising trials.
While the probability of survival after radiation therapy alone was about 10 % in the 1960s, an average of more than 80 % of patients with local disease, meaning without visible metastases [see metastasis], can achieve long-term cure by a combination of local and chemotherapy today. Favourable prognosis usually requires complete tumour removal and good response to the chemotherapy that precedes surgery.
Patients with metastasised disease at primary diagnosis still have an unfavour-able prognosis despite of intensive chemotherapy (5-year survival rates are an average of about 20-25 %). Prognosis is more favourable for patients with single lung metastases that can be surgically removed, when compared to patients with bone or bone marrow metastases. Prognosis for patients with recurrent disease is similarly unfavourable. Most unfavourable is the prognosis for patients who develop early metastases after intensive frontline treatment. Currently active and future trials should help to improve the outcome of those patients as well.
Note: The survival rates mentioned in the text above are statistical values. Therefore, they only provide information on the total cohort of patients with osteosarcoma. They do not predict individual outcomes. In the context of cancer, the term „cure“ should rather be referred to as „free of cancer“, be-cause current treatment regimens may help to destroy the tumour, but they are also frequently associated with numerous late-effects. Early detection and appropriate management of these long-term secondary effects typically require intensive rehabilitation and thorough long-term follow-up care, although a patient may have been „cured“ from the cancer.
References 
- Zöllner SK, Amatruda JF, Bauer S, Collaud S, de Álava E, DuBois SG, Hardes J, Hartmann W, Kovar H, Metzler M, Shulman DS, Streitbürger A, Timmermann B, Toretsky JA, Uhlenbruch Y, Vieth V, Grünewald TGP, Dirksen U: Ewing Sarcoma-Diagnosis, Treatment, Clinical Challenges and Future Perspectives. Journal of clinical medicine 2021 Apr 14; 10 [PMID: 33919988]
- Erdmann F, Kaatsch P, Grabow D, Spix C: German Childhood Cancer Registry - Annual Report 2019 (1980-2018). Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz 2020 [URI: https://www.kinderkrebsregister.de/ typo3temp/ secure_downloads/ 42507/ 0/ 1c5976c2ab8af5b6b388149df7182582a4cd6a39/ Buch_DKKR_Jahresbericht_2019_komplett.pdf]
- Bauer S, Dirksen U, Schildhaus HU: [Systemic therapy of sarcomas : New biomarkers and therapeutic strategies]. Der Pathologe 2019, 40: 436 [PMID: 31243550]
- Dirksen U, Brennan B, Le Deley MC, Cozic N, van den Berg H, Bhadri V, Brichard B, Claude L, Craft A, Amler S, Gaspar N, Gelderblom H, Goldsby R, Gorlick R, Grier HE, Guinbretiere JM, Hauser P, Hjorth L, Janeway K, Jürgens H, Judson I, Krailo M, Kruseova J, Kuehne T, Ladenstein R, Lervat C, Lessnick SL, Lewis I, Linassier C, Marec-Berard P, Marina N, Morland B, Pacquement H, Paulussen M, Randall RL, Ranft A, Le Teuff G, Wheatley K, Whelan J, Womer R, Oberlin O, Hawkins DS, Euro-EWING 99 and Ewing 2008 Investigators: High-Dose Chemotherapy Compared With Standard Chemotherapy and Lung Radiation in Ewing Sarcoma With Pulmonary Metastases: Results of the European Ewing Tumour Working Initiative of National Groups, 99 Trial and EWING 2008. Journal of clinical oncology 2019,JCO1900915 [PMID: 31553693]
- Whelan J, Le Deley MC, Dirksen U, Le Teuff G, Brennan B, Gaspar N, Hawkins DS, Amler S, Bauer S, Bielack S, Blay JY, Burdach S, Castex MP, Dilloo D, Eggert A, Gelderblom H, Gentet JC, Hartmann W, Hassenpflug WA, Hjorth L, Jimenez M, Klingebiel T, Kontny U, Kruseova J, Ladenstein R, Laurence V, Lervat C, Marec-Berard P, Marreaud S, Michon J, Morland B, Paulussen M, Ranft A, Reichardt P, van den Berg H, Wheatley K, Judson I, Lewis I, Craft A, Jürgens H, Oberlin O, Euro-EWING99 and EWING-2008 Investigators: High-Dose Chemotherapy and Blood Autologous Stem-Cell Rescue Compared With Standard Chemotherapy in Localized High-Risk Ewing Sarcoma: Results of Euro-E. W.I. N.G. 99 and Ewing-2008. J Clin Oncol 2018 [PMID: 30188789]
- Scobioala S, Ranft A, Wolters H, Jabar S, Paulussen M, Timmermann B, Jürgens H, Hassenpflug W, Klingebiel T, Elsayad K, Eich HT, Dirksen U: Impact of Whole Lung Irradiation on Survival Outcome in Patients With Lung Relapsed Ewing Sarcoma. International journal of radiation oncology, biology, physics 2018 Nov 1; 102: 584 [PMID: 30244879]
- Whelan J, Hackshaw A, McTiernan A, Grimer R, Spooner D, Bate J, Ranft A, Paulussen M, Jürgens H, Craft A, Lewis I: Survival is influenced by approaches to local treatment of Ewing sarcoma within an international randomised controlled trial: analysis of EICESS-92. Clinical sarcoma research 2018, 8: 6 [PMID: 29610659]
- Dirksen U, Jürgens H: Ewing-Sarkom. in: Niemeyer C, Eggert A (Hrsg.): Pädiatrische Hämatologie und Onkologie, Springer-Verlag GmbH Deutschland, 2. vollständig überarbeitete Auflage 2018, 497 [ISBN: 978-3-662-43685-1]
- Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, Delattre O, Paulussen M, Picci P, Sundby Hall K, van den Berg H, Ladenstein R, Michon J, Hjorth L, Judson I, Luksch R, Bernstein ML, Marec-Bérard P, Brennan B, Craft AW, Womer RB, Jürgens H, Oberlin O: Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015 Sep 20; 33: 3036 [PMID: 26304893]
- Haeusler J, Ranft A, Boelling T, Gosheger G, Braun-Munzinger G, Vieth V, Burdach S, van den Berg H, Jürgens H, Dirksen U: The value of local treatment in patients with primary, disseminated, multifocal Ewing sarcoma (PDMES). Cancer 2010, 116: 443 [PMID: 19924786]
- Burkhardt B, Reiter A, Landmann E, Lang P, Lassay L, Dickerhoff R, Lakomek M, Henze G, von Stackelberg A: Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: a report from the berlin-frankfurt-muenster group. Journal of clinical oncology 2009, 27: 3363 [PMID: 19433688]
- Gerth HU, Juergens KU, Dirksen U, Gerss J, Schober O, Franzius C: Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2007, 48: 1932 [PMID: 18006618]
- Wagner LM, McAllister N, Goldsby RE, Rausen AR, McNall-Knapp RY, McCarville MB, Albritton K: Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatric blood & cancer 2007, 48: 132 [PMID: 16317751]
- Hunold A, Weddeling N, Paulussen M, Ranft A, Liebscher C, Jürgens H: Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatric blood & cancer 2006, 47: 795 [PMID: 16411206]
- Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, Jürgens H: Ewing's sarcoma family of tumors: current management. The oncologist 2006, 11: 503 [PMID: 16720851]
- Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU 3rd, Eary JF: [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005, 23: 8828 [PMID: 16314643]
- Dirksen U, Poremba C, Schuck A: Knochentumoren des Kindes-und Jugendalters. Der Onkologe 2005, 10: 1034
- Schuck A, Ahrens S, Paulussen M, Kuhlen M, Konemann S, Rube C, Winkelmann W, Kotz R, Dunst J, Willich N, Jürgens H: Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int J Radiat Oncol Biol Phys 2003, 55: 168 [PMID: 12504050]


 Brief information on Ewing Sarcoma (481KB)
Brief information on Ewing Sarcoma (481KB)