Acute Lymphoblastic Leukaemia (ALL) – Brief Information
Acute lymphoblastic leukaemia (ALL), also known as acute lymphatic leukaemia, is a malignant disease of the bone marrow. This text provides information about the characteristics and subtypes of the disease, its frequency, causes, symptoms, diagnoses, treatment, and prognosis.
Author: Maria Yiallouros, Editor: Maria Yiallouros, Reviewer: Prof. Dr. med. Günter Henze, Dr. med. Anja Möricke, PD Dr. med. Gabriele Escherich, English Translation: Dr. med. Gesche Riabowol (nee Tallen), Hannah McRae, Last modification: 2024/02/27 https://dx.doi.org/10.1591/poh.patinfo.all.kurz
Table of contents
General disease information
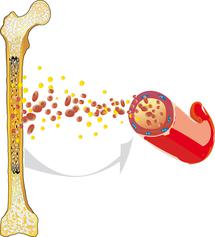
Acute lymphoblastic leukaemia (ALL) is a malignant cancer that arises within the haematopoietic system. ALL usually originates from the bone marrow, where the blood cells are produced. It is characterised by an overproduction of impaired white blood cells.
Healthy blood cells reproduce and regenerate at a normal, balanced rate. They undergo a complex maturation process. ALL interferes with this process: The white blood cells (leukocytes) are unable to mature into functional cells and instead multiply rapidly and uncontrollably. This disturbs normal blood cell formation, so that healthy white blood cells, red blood cells (erythrocytes), and platelets (thrombocytes) can no longer be produced to the extent that is necessary.
Lack of red blood cells (anaemia), infections, and bleeding tendencies can result and may be the first signs of acute leukaemia. Since ALL is not limited to one specific region of the body, but can spread from the bone marrow into the blood and the lymphatic system, it can affect all organs and organ systems and is, therefore (like all leukaemias), known as a malignant systemic disease.
ALL progresses rapidly. The spread of leukaemia cells and the resulting damage to other body parts can cause serious diseases, which – without the appropriate Treatment – are lethal within a few weeks or months.
Incidence
Comprising about 80 % of childhood leukaemias, acute lymphoblastic leukaemia (ALL) is the most common form of leukaemia in children and adolescents. It accounts for approximately 22 % (one fifth) of all cancers in this age group. According to the German trial centres, about 550 to 600 children and adolescents aged 0 to 17 years are newly diagnosed with ALL in Germany each year. In general, ALL can occur at any age, including adults. However, the frequency of ALL is highest in children between 1 and 5 years of age, with boys being slightly more affected than girls (gender ratio: 1.3 to 1).
Types of ALL
ALL is mainly characterised by a malignant transformation of immature precursor cells of lymphocytes. This transformation can occur during every stage of cell development (differentiation), thereby affecting various subtypes of lymphocytes as well as their precursors. For this reason, there are various forms of ALL. So-called B-ALL subtypes, for example, are based on progenitor cells of B lymphocytes, while T-ALL forms from precursors of T lymphocytes. A degeneracy in the early development stages is characterised by the prefix "pre" or “pro”.
This results in the following ALL-subtypes:
- Pro-B-ALL (also pre-pre-B-ALL)
- Common ALL
- Pre-B-ALL
- Mature B-ALL (or B-AL)
- Pro-T-ALL
- Pre-T-ALL
- Cortical (intermediate) T-ALL
- Mature T-ALL
The classification of the ALL subtypes listed above is based on the results of a special laboratory test (immunophenotyping). An even more precise characterisation can be done based on the genetic features of the leukaemia cells. It is important to know that there are multiple forms of ALL, because when it comes to the course and prognosis of this disease, there are differences between each type to some extent. These differences are considered during the selection of a treatment plan.
Causes
The causes of acute lymphoblastic leukaemia (ALL) are largely unknown. It is known so far that the disease arises from the malignant transformation of precursor lymphocytes, and also, that this transformation can be associated with genetic alterations of these cells. Why these genetic alterations exist and why they cause the disease in some children but not in others, remains to be discovered.
For example, there is a known gene mutation in ALL that can be found in some newborns, even if they do not present with the disease until years later. Furthermore, not every child with this kind of genetic mutation will suffer from ALL. This suggests that, in addition to genetic and immunological factors, the immune system as well as environmental influences can play a role in pathogenesis. It seems that many factors must come together for ALL to occur.
It is also known that children with certain inherited or acquired immunodeficiencies [see immunodeficiency] as well as young patients with chromosomal alterations have a higher risk of developing acute leukaemia than their healthy peers. Hereditary concomitant diseases promoting the development of ALL are, for example, Down syndrome, Fanconi anaemia, Bloom syndrome, Louis-Bar syndrome (ataxia telangiectasia) and Li-Fraumeni syndrome. Since these (very rare) diseases are associated with a predisposition to develop cancer, they are also called cancer predisposition syndromes.
Also, exposure to radioactive radiation and X-rays, certain chemicals and drugs as well as certain viruses have been reported to play a role in the development of leukaemia. However, for most patients no specific cause for the development of ALL can be identified.
Symptoms
The health problems (symptoms) caused by ALL usually develop within only a few weeks. They mainly occur due to the increase of malignant cells within the bone marrow as well as their spread into other organs and tissues. The uncontrolled production of leukaemia cells in the bone marrow increasingly suppresses the production of normal blood cells.
Children and adolescents suffering from ALL initially experience general symptoms such as fatigue, pain, and pallor. This is due to the lack of red blood cells (anaemia), the function of which it is to carry oxygen to cells throughout the body. The lack of functional white blood cells (i.e. lymphocytes and granulocytes) prevents pathogens from being attacked and eliminated properly, thereby causing infections and fever. Another frequent symptom is bleeding, for example, under the skin (bruises, petechiae) or from mucous membranes such as the gums, owing to impaired blood coagulation as a result of low platelet counts.
The growth of leukaemia cells in the marrow of the long bones can cause bone and joint pain, especially in the limbs (arms and legs) and back. This pain can be so intense that the affected child may refuse to walk or run. The malignant cells can also spread into the liver, spleen, and lymph nodes. Therefore, these organs may enlarge and subsequently cause problems, such as abdominal pain. In general, all organs can be affected by ALL. If ALL spreads to the brain and its meninges, patients may suffer from headache, visual disturbances, nausea, vomiting, and other central nervous system impairments
The most important symptoms are summarized as follows:
- fatigue, exhaustion, weariness, malaise
- pallor (caused by the lack of red blood cells, anaemia)
- increased risk of bleeding, for example, frequent spontaneous nose bleeding, gum bleeding (for example while brushing teeth), excessive bruising, pinpoint, round and red spots on the skin (petechiae); rare: cerebral haemorrhage (due to lack of platelets)
- fever and/or frequent nonspecific infections (due to the lack of white blood cells, neutropenia)
- enlarged lymph nodes (for example, in neck, armpits, groin)
- abdominal pain and loss of appetite (due to the enlargement of spleen and/or liver)
- bone and joint pain (mostly limbs and back)
- headache, visual disturbances, vomiting, cranial nerve palsies (if the central nervous system is involved)
- shortness of breath (due to the enlargement of the thymus gland or of lymph nodes in the chest area)
- enlargement of testicle(s)
Good to know: The type and degree of symptoms of ALL vary individually. It is also important to know that the occurrence of one or more of these symptoms does not necessarily mean that they are caused by leukaemia. Many of these symptoms also occur in benign diseases that have nothing to do with leukaemia. However, if these symptoms occur or recur frequently or persist, a doctor should be consulted as soon as possible. If acute leukaemia is diagnosed, treatment must be started promptly.
Diagnosis
If the doctor, based on the young patient’s history (anamnesis) and physical examination, suspects acute leukaemia, he or she will first initiate a blood test. If the results promote the diagnosis of an acute leukaemia, a sample of the bone marrow (bone marrow puncture) is required for confirmation. For bone marrow tests and other diagnostice procedures, the doctor will refer the patient to a children's hospital with a paediatric oncology program (paediatric oncology unit).
Blood and bone marrow tests
Blood and bone marrow tests are needed to confirm the diagnosis of leukaemia as well as to determine the type. The tests include microscopic (cytomorphological), immunological and genetic laboratory analysis of blood and bone marrow samples that distinguish ALL from other kinds of leukaemia (such as AML) and, furthermore, allow to define the specific subtype of ALL. Knowing the subtype of ALL is necessary for appropriate therapy planning, because different forms of ALL have different cellular and molecular characteristics and they also vary regarding their response to treatment and, thus, prognosis.
Tests to assess spread of the disease (staging)
Following the diagnosis of ALL and its subtype, it is important for treatment planning to know whether the leukaemia cells have spread to additional body compartments (other than the bone marrow), including the brain, liver, spleen, lymph nodes, testicles, or bones. Therefore, various imaging techniques, such as ultrasound, X-ray examination, magnetic resonance imaging (MRI) and computed tomography (CT), may be used to evaluate spread of the disease. To find out whether the central nervous system (brain and spinal cord) is affected, a sample of cerebrospinal fluid is taken and analysed for leukaemia cells (lumbar puncture).
Additional diagnostics before treatment begins
For treatment preparation, tests on the patient's cardiac function (electrocardiography [ECG] and echocardiography) and brain function (electroencephalography, EEG) are performed. Furthermore, additional bloodwork is needed to assess the patient's general health condition and to determine the patient's blood group (essential in case a blood transfusion may be necessary during the course of treatment). Also, the functions of certain organs (such as kidneys and liver) need to be evaluated by blood tests in order to rule out potential metabolic disorders that can have negative impact on the treatment. Having collected all this information prior to treatment helps the doctors later to detect and thus treat treatment-induced changes earlier.
Good to know: Not all the tests listed above need to be done for every patient. Contrariwise, the patient’s individual situation may require additional diagnostic procedures that have not been mentioned in this chapter. Therefore, you should always ask your doctor, based on the information above, which test your child needs and why.
Treatment planning
After the diagnosis has been confirmed, therapy is planned. In order to design an individual, risk-adapted treatment regimen for the patient, certain factors influencing the patient’s chance of recovery (prognosis) – called risk factors or prognostic factors – are being considered during treatment planning (risk-adapted treatment strategy).
Important prognostic factors are the subtype of ALL (see chapter „Types of ALL“), the extent of leukemia cell spread throughout the body at diagnosis, the response of the disease to treatment, and the patient’s age. The exact knowledge of the tumour type helps the caregiver team to assess how sensitive the tumour cells might be to the chemotherapy and thus, how intensely patients need to be treated in order to keep the risk of relapse as low as possible. Extent of the disease and response to treatment impact the decision whether, aside from chemotherapy, additional treatment methods (for example cranial radiation or high-dose chemotherapy followed by stem cell transplantation) are necessary to increase the probability of cure.
All these factors are included in treatment planning in order to achieve the best outcome possible for each patient. Hence, each individual clinical situation is crucial for assigning a patient to a treatment group (standard risk, medium risk, high risk group) and for choosing the optimal treatment protocol.
Treatment
Treatment of children and adolescents with acute lymphoblastic leukaemia (ALL) should take place in a children's hospital with a paediatric oncology program. Only in such a treatment centre, highly experienced and qualified staff (doctors, nurses and many more) is guaranteed, since they are specialised and focus on the diagnostics and treatment of children and teenagers with cancer according to the most advanced treatment concepts. The doctors (such as oncologists, radiologists, surgeons) in these centres collaborate closely with each other. Together, they treat their patients according to treatment plans (protocols) that are continuously optimised. The goal of the treatment is to achieve high cure and low rates of side effects.
Treatment methods
- Chemotherapy is the major backbone of ALL treatment. It uses drugs (so-called cytostatic agents) that can kill fast-dividing cells, such as cancer cells, or inhibit their growth, respectively. Since one cytostatic agent alone may not be capable of destroying all the leukaemia cells, a combination of cytostatics that function in different ways are usually given (polychemotherapy).
- Radiation therapy of the brain (cranial irradiation) is used for some patients in addition to chemotherapy. However, radiation therapy (radiotherapy) is increasingly reduced and hence its indication currently subject to change.
- Stem cell transplantation: For some patients, high-dose chemotherapy (and, partly, total body irradiation) followed by stem cell transplantation may be an option.
The goal of treatment is to eliminate the leukaemia cells in the body as extensively as possible, so that the bone marrow can resume its function as a blood cell-producing organ. The intensity and duration of chemotherapy, the need for radiotherapy and/or stem cell transplantation, as well as the prognosis of the disease, depend on the subtype of ALL, on how extensively the leukaemia cells have spread throughout the body, whether the patient tolerates the treatment, and whether the leukaemia responds to it (see chapter “treatment planning”). In order to prevent or adequately manage the side effects of the intensive therapy, specific supportive care regimens have been established and now represent an important and efficient component of ALL treatment.
Note for patients with mature B-ALL (B-AL): This ALL subtype is not treated like the other forms of ALL. Patients with mature B-ALL will receive a treatment similar to that of mature B-cell Non-Hodgkin lymphoma and are therefore not included in the following chapters. Information on Non-Hodgkin lymphoma can be found here.
Course of treatment
Treatment of children and teenagers with ALL consists of different steps. These steps (or phases) have different purposes. Therefore, they vary regarding their duration, treatment intensity, and drug combinations. Each treatment period follows a different treatment plan (protocol), and each treatment plan is adapted to the patient's individual situation. The protocol the doctors decide to use for a patient depends, for example, on the subtype of ALL, the results of the staging, and other individual factors that are important for the patient's risk of recurrent disease. As a rule of thumb, the doctors will recommend more intense treatment if your child has a relatively high risk of relapse.
Usually, treatment will take about a total of two years, in particular if the disease responds sufficiently to therapy and no relapse occurs. There will be both times spent as inpatient (about six months total with discharges in between) and outpatient (about one and a half years).
The major elements of ALL treatment are:
- Pretreatment (preliminary phase): In many treatment protocols for ALL, induction therapy begins with the so-called cytoreductive pre-phase. It serves to initiate therapy and consists of a short, approximately one-week long, phase of chemotherapy using moderate dosages of one or two different agents. The purpose of this pretreatment is to reduce the often initially heavy burden of leukaemia cells gradually. This relatively gentle start helps the doctors to keep the metabolic products released by the dying leukaemia cells under control, which is important, because such metabolites can seriously harm the patient's organs, especially the kidneys (so-called tumour lysis syndrome).
- Induction therapy: The actual induction therapy consists of an intense phase of chemotherapy using a combination of different agents. It aims at eliminating most of the leukaemia cells, thereby inducing remission in a short period of time. Induction therapy usually takes about five to eight weeks.
- Consolidation / intensification therapy: The consolidation or intensification therapy is also an intensive chemotherapy, partially using different combinations of agents and higher dosages. The goal is to consolidate the remission achieved by induction therapy by further eliminating leukaemia cells in order to reduce the risk of recurrent disease. The intensity of treatment is based on the patient’s individual risk of relapse; accordingly, this treatment phase may take between weeks and months. It is also designed to reach the central nervous system (CNS) and the testes (so-called extracompartment therapy; see below).
- CNS therapy (extracompartment therapy): Major part of all the intensive treatment phase, in particular of the consolidation/intensification phase, is the treatment of the central nervous system (CNS). The CNS therapy is extremely important, since ALL affects the CNS in most patients, even if no leukaemia cells are detectable in the cerebrospinal fluid. Most chemotherapeutic agents cannot pass the blood-brain barrier. Therefore, treatment is performed using high doses of certain agents that can pass this barrier due to their specific characteristics. Also, chemotherapy is given directly into the spinal canal via lumbar puncture (intrathecal chemotherapy). For some (few) patients, cranial radiotherapy may become an option, for example, if central nervous system involvement has been proven.
- Reinduction therapy: Aside from the treatment phases described above, another intensification strategy, the reinduction therapy, has proven to be an effective approach in ALL patients. Reinduction aims at completely destroying leukaemia cells, thereby reducing the risk of recurrent disease as much as possible. The reinduction phase can last between weeks and months depending on the risk group the patient has been assigned to. In case of longer treatment times, for example for high-risk patients, short intensive phases of therapy alternate with more moderate ones in order to ensure the patient’s recovery between courses.
- Maintenance therapy: This last phase of treatment is designed to eliminate all the leukaemia cells that may not be detectable but still have survived despite intensive treatment. The intensity of chemotherapy is much less than in the other phases. Also, the patient is mainly outpatient and may even continue with kindergarten or school. This phase of treatment is usually continued until a total treatment time of two years has been achieved.
Typical cytostatics in ALL treatment are, for example, prednisone (PRED) or dexamethasone (DEXA), vincristine (VCR), daunorubicin (DNR), asparaginase (ASP), methotrexate (MTX), cyclophosphamide (CPM), cytarabine (ARA-C), 6-mercaptopurine (6-MP), etoposide (VP-16), and thioguanine (TG).
Note: Depending on the trial, the terminology of the different treatment phases as well as their duration and design may vary. Also, new treatment approaches (such as immunotherapy with antibodies) may be examined in the context of clinical studies.
Therapy in case of recurrent disease (relapse)
In case of recurrent disease (relapse), which occurs in about 15 % of ALL patients, treatment options consist of chemotherapy, radiation therapy, and stem cell transplantation. The majority of patients receive an intensified chemotherapy regimen aiming at remission, followed by an allogeneic stem cell transplantation. In some patients, remission may be achieved by chemotherapy only. Radiotherapy is given in case of central nervous system and/or testicular involvement. Patients whose disease does not respond to conventional treatment approaches (refractory or secondary ALL relapse), may benefit from new substances with different mechanisms of action, which are currently being investigated in various clinical trials.
Therapy optimising trials and registries
In Germany, diagnosis and treatment of almost all children and adolescents with acute lymphoblastic leukaemia (ALL) are performed according to the treatment plans (protocols) of "therapy optimising trials", so named because the treatment concepts of such trials are continuously being optimised based on the latest medical knowledge and the experience with former protocols. Therapy optimising trials are standardised and controlled studies that aim at steadily developing and improving treatment possibilities for cancer patients. They are usually applied in numerous treatment centres, not only in Germany but also abroad (multicentric and international studies).
Patients who are not included in any trial, either because they suffer disease while there is no trial available or because they do not, for some reason, fit into one of the existing trials, are often included in so-called registries. The patients are generally treated according to the recommendations of the trial centre, thus receiving the current best therapy available.
Currently, the following therapy optimising trials are available for children and adolescents with ALL in Germany:
- Trial AIEOP-BFM ALL 2017: international therapy optimising trial for the treatment of children and adolescents aged 0 to 17 years with first diagnosis of ALL. In contrast to the preceding trial (AIEOP-BFM ALL 2009), this active trial is also recruiting children in their first year of life. Multiple paediatric oncology centres in Germany, other European countries, and Australia are participating in this trial, which was opened mid 2018. The German study centre is located at the University Hospital (Universitätsklinikum) Schleswig-Holstein, Campus Kiel (Principal Investigator: Prof. Dr. med. Martin Schrappe).
- Trial ALLTogether1: international therapy optimising study of the newly founded European consortium ALLTogether for children, adolescents and adults (between 0 and 45 years of age) with newly diagnosed ALL. The worldwide largest study group, which also includes the CoALL study centre located in Hamburg (chair: PD Dr. med. Gabriele Escherich), is examining new treatment concepts considering therapy intensification as well as therapy reduction based on the patient’s individual risk of relapse and by using multiple randomization arms. In Germany, the ALLTogether1 trial has been open since April 1, 2022 (principal investigator: Mats Marshall Heyman, MD, PhD, Karolinska University Hospital, Stockholm). The German chair is PD Dr. med. Gabriele Escherich (CoALL study centre) at Universitätsklinikum Hamburg-Eppendorf.
- Registry CoALL 2020 (CoALL stands for Cooperative ALL Trial): children and adolescents under the age of 18 who are not treated within the ALLtogether1 trial can be registered with the CoALL 2020 Registry. The registry accepts children and adolescents with any type of ALL since May 2020 (as a transitory solution after study COALL-08-09 was closed for patient recruitment). It also includes patients with ALL as a secondary malignancy as well as with non-Hodgkin lymphoma. Therapy recommendations within the registry correspond to the standard therapy of the ALLTogether trial. Chair of registry and study centre is PD Dr. med. Gabriele Escherich (MD, PhD), Universitätsklinikum Hamburg-Eppendorf.
- Trial EsPhALL2017 / COGAALL1631: international, multicentric therapy optimising trial for the treatment of children and adolescents between 1 and 21 years of age with Philadelphia chromosome-positive ALL. In Germany, the trial has been open for recruitment since November 15, 2019. The German study centre is located at Universitätsklinikum Schleswig-Holstein (Campus Kiel); the principal investigator is Prof. Dr. med. G. Cario.
- Registry Interfant-06: registry for infants in the first year of life with ALL or biphenotypic leukaemia (subgroup of ALL). The register is sequel to the trial INTERFANT-06, which was closed for patient admission in October 2016. The German trial centre is located at the University Hospital (Universitätsklinikum) Schleswig-Holstein, Campus Kiel, under the supervision of Prof. Dr. med. Martin Schrappe. A new international study (INTERFANT 21) will most certainly be opened by the first quarter of 2024 with PD Dr. med. Gabriele Escherich (MD, PhD) as the chair at Universitätsklinikum Hamburg-Eppendorf.
- Registry ALL SCT FORUM 2022 : national registry for patients (under 21 years of age) with a high or very high risk of relapse, for whom allogeneic stem cell transplantation is an option. The interim registry has been open since 13/09/2022, being sequel to the trial ALL SCTped 2012 FORUM (which was closed in 2022). Transplant centres throughout Germany are participating. The study director in charge is Prof. Dr. med. Peter Bader at the Johann-Wolfgang-Goethe University Hospital, Frankfurt.
- Trial IntReAll HR 2010: international, multicentric therapy optimising trial for children and adolescents (under 18 years of age) with a first high-risk relapse of ALL (B-precursor- or T-cell-ALL, high-risk patients). Numerous paediatric oncology centres throughout Germany as well as in other European and non-European countries participate in this trial. The study centre is located at the Department of Paediatric Oncology and Haematology of the Charité Berlin (Study Director: PD Dr. Arend von Stackelberg).
- Observation Trial ALL-REZ: this trial collects data of recurrent disease patients (under 18 years of age) that are not included in above-mentioned study, such as children and adolescents with a second relapse or a first but high-risk relapse of ALL (principal investigator: PD Dr. Arend von Stackelberg, Department of Paediatric Oncology and Haematology of the Charité Berlin).
Please note: trial AIEOP-BFM ALL 2017 and CoALL trial / registry are designed for the same group of patients (patients with newly-diagnosed ALL, aged 0 to 17 or 1 to 17, respectively) and only differ but slightly. The choice between the two trial protocols or resulting recommendations, respectively, is made by the local treatment team, depending on which of the two protocols the respective treatment centre is spezialised in. Since April 2022, the CoALL study group – as one of nine study groups in total – has been participating in the new phase III study of the international ALLTogether consortium.
Please note that patients with mature B-ALL (B-AL) are not considered here, since they receive a treatment for mature B-cell Non-Hodgkin lymphoma.
The major goal of therapy optimising trials is to continuously improve the treatment and, thus, the outcome of all ALL patients and to minimise treatment-related side effects. The experience with a previous trial will be incorporated into the subsequent protocol, thereby providing continuous optimisation and knowledge gain.
Prognosis
The chances of cure (prognosis) for children and adolescents with acute lymphoblastic leukaemia (ALL) have significantly improved due to the immense progress in diagnostics and treatment over the last four decades. Today’s modern diagnostic procedures and the use of intensive, standardised polychemotherapy protocols combined with optimised supportive care regimens currently result in 10-year survival rates of about 90 %. Hence, ALL is among the best treatable malignancies.
However, for children with unfavourable prognostic factors, such as nonresponse to therapy and/or certain hard-to-treat ALL subtypes, survival rates are considerably lower than 90 %. That also applies to patients who are either under one year of age or over ten years old.
About 90 of the 550 to 600 children and adolescents (approximately one in seven patients) newly diagnosed with ALL in Germany per year develop recurrent disease (relapse). Recurrent disease most frequently appears during the first two to three years after the initial diagnosis, while it is rather rare after five years following first diagnosis of ALL. The prognosis is generally worse than during the initial treatment, although in a subset of patients treatment success can still be achieved. The 5-year survival rates in children and adolescents with ALL relapse are currently at about 50 to 60 %. Under the current therapy optimising trials and future studies, the chances of cure are continually improved for these patients.
Note: The survival rates mentioned in the text above are statistical values. Therefore, they only provide information on the total cohort of patients with childhood ALL. They do not predict individual outcomes. Acute leukaemias can show unpredictable courses, in both patients with favourable and patients with unfavourable preconditions.
 PDF Brief Information on Acute Lymphoblastic Leukaemia (ALL) (477KB)
PDF Brief Information on Acute Lymphoblastic Leukaemia (ALL) (477KB)
Author: Maria Yiallouros
26/01/2024
References 
- Stanulla M, Erdmann F, Kratz CP: Risikofaktoren für Krebserkrankungen im Kindes- und Jugendalter. Monatsschrift Kinderheilkunde 169, 30-38 2021 [DOI: 10.1007/s00112-020-01083-8]
- Escherich G, Schrappe M, Creutzig U: Akute lymphoblastische Leukämie – ALL – im Kindesalter. AWMF online 2021 [URI: https://www.awmf.org/ uploads/ tx_szleitlinien/ 025-014l_S1_Akute-lymphoblastische-Leukaemie-ALL-im-Kindesalter_2021-07.pdf]
- Erdmann F, Kaatsch P, Grabow D, Spix C: German Childhood Cancer Registry - Annual Report 2019 (1980-2018). Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz 2020 [URI: https://www.kinderkrebsregister.de/ typo3temp/ secure_downloads/ 42507/ 0/ 1c5976c2ab8af5b6b388149df7182582a4cd6a39/ Buch_DKKR_Jahresbericht_2019_komplett.pdf]
- Schrappe M, Möricke A, Attarbaschi A, von Stackelberg A: Akute lymphoblastische Leukämie. in: Niemeyer C, Eggert A (Hrsg.): Pädiatrische Hämatologie und Onkologie. Springer-Verlag GmbH Deutschland, 2. vollständig überarbeitete Auflage 2018, 269 [ISBN: 978-3-662-43685-1]
- Tallen G, Henze G, von Stackelberg A: Treatment of children and adolescents with relapsed ALL: therapy target long-term healing. Pharm Unserer Zeit 2012, 41: 214 [PMID: 22844668]
- Möricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, Ludwig WD, Ritter J, Harbott J, Mann G, Klingebiel T, Zintl F, Niemeyer C, Kremens B, Niggli F, Niethammer D, Welte K, Stanulla M, Odenwald E, Riehm H, Schrappe M: Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia : 2010, 24: 265 [PMID: 20010625]
- Escherich G, Horstmann MA, Zimmermann M, Janka-Schaub GE, COALL study group: Cooperative study group for childhood acute lymphoblastic leukaemia (COALL): long-term results of trials 82,85,89,92 and 97. Leukemia : 2010, 24: 298 [PMID: 20016530]
- Schrappe M: Risk-adapted stratification and treatment of childhood acute lymphoblastic leukaemia. Radiation protection dosimetry 2008, 132: 130 [PMID: 19017727]
- Schrauder A, von Stackelberg A, Schrappe M, Cornish J, Peters C, ALL-BFM Study Group, EBMT PD WP, I-BFM Study Group: Allogeneic hematopoietic SCT in children with ALL: current concepts of ongoing prospective SCT trials. Bone marrow transplantation 2008, 41 Suppl 2:S71 [PMID: 18545248]
- Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dördelmann M, Löning L, Beier R, Ludwig WD, Ratei R, Harbott J, Boos J, Mann G, Niggli F, Feldges A, Henze G, Welte K, Beck JD, Klingebiel T, Niemeyer C, Zintl F, Bode U, Urban C, Wehinger H, Niethammer D, Riehm H, Schrappe M, German-Austrian-Swiss ALL-BFM Study Group: Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008, 111: 4477 [PMID: 18285545]